
Starting Discovery with the Right Analytics during rAAV Process Development Assures Confidence in Your Final Product!
QA provides the foundation for safety, consistency, and compliance, making it an integral part of any successful rAAV discovery and process development strategy. Achieving excellence in Quality assurance depends on selecting the appropriate analytical tools.
At NewBiologix, our analytics solutions provide confidence in final product integrity, addressing regulatory agencies' demands for answers to gene therapy safety.
Our analytics platform vigilantly monitors quality at every process step detecting contaminants and assessing gene integrity from cell lysis to formulation.
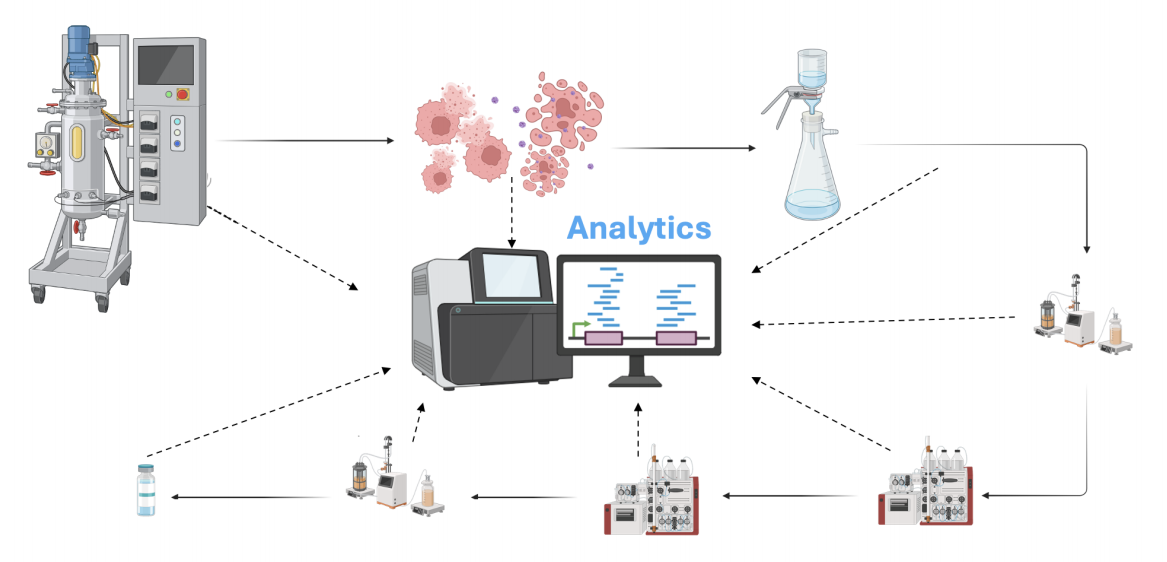
The road to successful CMC packages
Quality of rAAV Encapsidated DNA analysis during Process Development
1. Starting with mass photometry to identify full-to-empty capsid ratios, we accelerate decision-making, enhance process insights, and ensure production of high-quality, consistent vectors.
2. We have developed in-house methods using Multidimensional PCR, rapidly emerging as a next-generation standard in rAAV analytics. This approach complements traditional qPCR and ddPCR by delivering greater depth, resolution, and interpretability, with a particular focus on genome integrity, a critical factor for clinical efficacy and regulatory approval.
3. Next Generation Sequencing (NGS) off ers the most comprehensive genomic characterization of rAAV vectors available today. It is essential for precisely understanding what is being delivered to the patient, not only the quantity of vectors, but the exact composition. For regulatory submissions or quality control packages, NGS data adds depth, strengthens the dossier and mitigates risk during regulatory review.
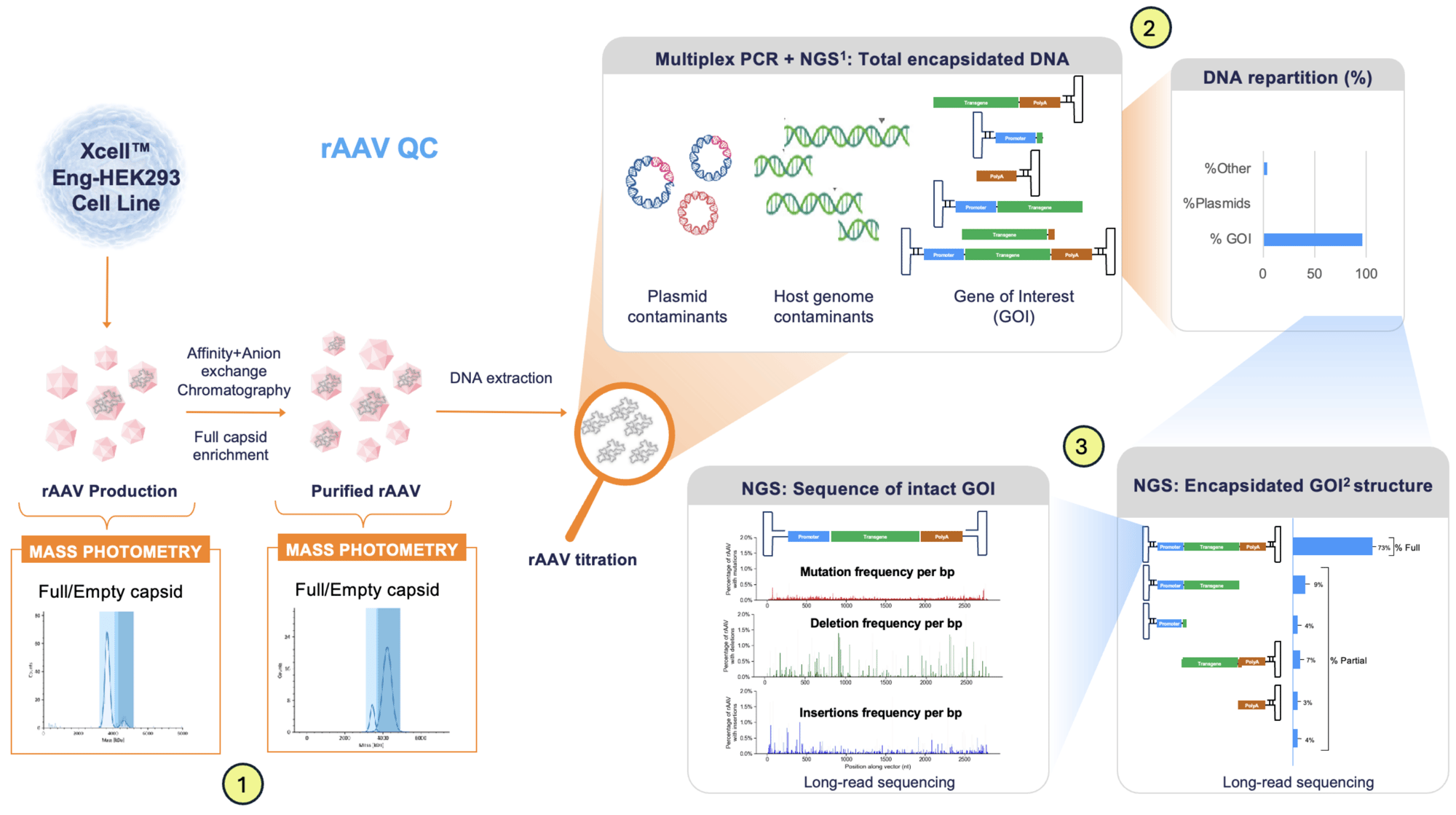
The combined use of Multidimensional PCR and NGS gives both precision and depth:
- Multidimensional PCR: Fast, reliable QC tool for critical regions.
- NGS: Comprehensive, base-level genomic auditing.
Together, they enable robust product understanding, meet regulatory expectations, and ensure the development of safe, high-quality rAAV vectors.
Why Quality Assurance Matters
In rAAV production, quality assurance (QA) is not optional, it's the foundation for clinical success, regulatory approval, and patient trust. At NewBiologix, we provide the essential tools to establish robust cGMP CMC quality systems and processes that stage for safe, consistent and compliant vector manufacturing.
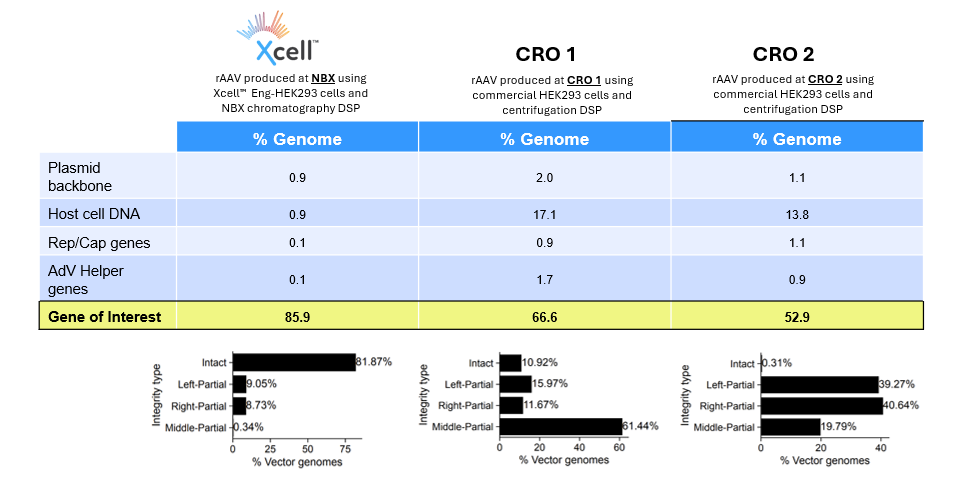
THE MORE INTACT ENCAPSIDATED DNA, THE HIGHER THE POTENCY!
To demonstrate the superior quality of viral particles produced for a Discovery (pre-IND) application using NewBiologix’s Xcell™ Eng-HEK293 cell line and chromatography-based purification process, we conducted a comparative analysis against rAAV vectors generated using a commercial HEK293 cell line and (ultra-)centrifugation-based purification methods from two leading CROs (see table below)
Contact us today to explore how we can work together to help patients benefit from life-saving treatments through safer, scalable, cost-efficient rAAV process development.
